Radiometric dating accuracy
14.06.2017
radiometric dating accuracy

Before we can calculate the age of a rock from its measured chemical composition, we must assume what radioactive elements were in the rock when it formed. From the protective garment of skin to the engineering of our bones and new discoveries about our brain, this issue is packed with testimony to the Master Designer. This has led some to suggest that Earth's distance from the sun, which varies during the year and affects the planet's exposure to solar neutrinos, might be related to these anomalies. Obviously, if the substance you are measuring is contaminated, then all you know is the age since contamination, or worse, you don't know anything, because the contamination might be in the opposite direction - suppose, for example, you're looking at radio carbon carbon 14, which is produced in the atmosphere by cosmic rays, and which decays into nitrogen. Creation Today Tour of Creation Museum and Ark Encounter The Largest Memorial In The World Did the Dead Rise and Appear in Jerusalem? On the other hand, the concentration of carbon falls off so steeply that the age of relatively young remains can be determined precisely to within a few decades. Have any problems using the site? The only foolproof method for determining the age of something is based on eyewitness reports and a written record. An hourglass is a helpful analogy to explain how geologists calculate the ages of rocks. If you were radiometric dating accuracy to examine just one atom, you would not know whether or not it would decay. When the 14 C has been formed, like ordinary carbon 12 Cit combines with oxygen to give carbon dioxide 14 CO 2and radiometric dating accuracy it also gets cycled through the cells of plants and animals. From radiometric dating accuracy research, our evolutionary geologist may have discovered that other geologists believe that Sedimentary Rocks A are million years old and Sedimentary Rocks B are 30 million years old. But we do not have an instrument that directly measures age. This instability makes it radioactive. When an unstable Uranium U isotope decays, it turns into an isotope of the element Lead Pb. To derive ages from such measurements, unprovable assumptions have to be made such as:. For example, with regard to the volcanic lavas that erupted, flowed, and cooled to form rocks in the unobserved past, evolutionary geologists simply assume that none of the daughter argon atoms was in the lava radiometric dating accuracy.
Radiometric dating or radioactive dating is a technique used to date materials such as rocks or carbonin which trace radioactive impurities were selectively incorporated when they were formed. Sccuracy method compares the abundance of a naturally occurring radioactive isotope within the material to the abundance of its decay products, which form at a known constant rate of decay.
Together radiometric dating accuracy stratigraphic principlesradiometric dating methods are used in geochronology to establish the geological time scale. By allowing the establishment of geological timescales, it provides a significant source of information about the ages of fossils and the deduced rates of evolutionary change. Radiometric dating is also used to date archaeological materials, including ancient radiometric dating accuracy. Different radiometric dating accuracy of radiometric dating vary in the timescale over which they are accurate and the materials to which they can be applied.
All ordinary matter is made up of combinations of chemical elementseach with its own atomic numberindicating the number of protons in the atomic nucleus. Additionally, elements may exist in different radiometric dating accuracywith each isotope of an element differing in the number radiometrix neutrons in the nucleus. A rafiometric isotope of a particular element is called a nuclide. Some nuclides are inherently unstable. That is, at some point in time, an atom of such a nuclide will undergo radioactive decay and spontaneously transform into a different nuclide.
This transformation may be accomplished in a number radiomerric different ways, including alpha decay accudacy of alpha particles and beta decay electron emission, positron emission, or electron capture. Another possibility is spontaneous fission into two or more nuclides. While the moment in time at which a particular nucleus raxiometric is unpredictable, a collection of acvuracy of a radioactive nuclide decays exponentially at a rate described by a parameter known as radiometric dating accuracy half-lifeusually given in units of years when discussing dating techniques.
After one half-life has elapsed, one half of the atoms of the nuclide in question will have decayed into a "daughter" nuclide or decay product. In many cases, the daughter nuclide itself radiometrif radioactive, resulting in a decay chaineventually ending with the formation of a stable nonradioactive daughter nuclide; dtaing step in such a chain is characterized by a distinct half-life. Radiometric dating accuracy these cases, usually the half-life of interest in radiometric dating is the longest one in the chain, which is the rate-limiting factor in the ultimate transformation of the radioactive nuclide into datiing stable daughter.
Isotopic systems that have been exploited for radiometric dating have half-lives ranging from only about 10 years e. For most radioactive nuclides, the half-life depends solely on nuclear properties and is essentially a constant. It is not affected by external factors such radiometric dating accuracy temperaturepressurechemical environment, or presence of a magnetic or electric field. For all other nuclides, the proportion of the original nuclide gadiometric its decay products changes in a predictable way as the original nuclide decays over time.
This predictability allows the relative abundances of related nuclides to be used as a clock to measure radiometric dating accuracy time from the incorporation of the original nuclides into a material to the present. The basic equation of radiometric dating requires that neither the parent nuclide nor the daughter product can enter or leave the material after its formation. The possible confounding effects of contamination of parent and daughter isotopes have radiometric dating accuracy be considered, as do the effects of radiometric dating accuracy loss or gain radiometric dating accuracy such isotopes since the sample was created.
It is therefore essential to have as much information as possible about the material being dated and to check for possible signs of alteration. Alternatively, if several different minerals can be radiometric dating accuracy from the same sample and are assumed to be formed by the same event and were in equilibrium with the reservoir when they formed, they should form an isochron.
This can reduce the problem of contamination. In uranium-lead datingthe concordia diagram is used which also decreases the problem of nuclide radimetric. Finally, correlation between different isotopic dating methods may be required to confirm the accuraacy of a sample. For example, the age of the Amitsoq gneisses from western Greenland was determined to accracy 3. Accurate radiometric dating generally requires that the parent has a long radiometri half-life that it will be present in significant amounts at the time of measurement except as described below under "Dating with short-lived extinct radiometric dating accuracythe half-life of the wccuracy is accurately known, and enough of the daughter product is produced to be accurately measured and distinguished from the initial amount of the daughter present in the material.
The procedures used to isolate and analyze the parent and daughter nuclides must be precise and accurate. This radio,etric involves isotope ratio mass spectrometry. The precision of a dating method depends in part on the half-life of the radioactive radiometric dating accuracy involved. For instance, carbon has radiometriic half-life of 5, years.
After an organism has been dead for 60, years, so little carbon is left that accurate dating can not be established. On the other hand, the concentration of carbon falls off so steeply that the age of relatively young remains can be determined precisely to within a few decades. If a material that selectively accugacy the daughter nuclide is heated, any daughter nuclides that have been accumulated over time will be lost through diffusionsetting radiometric dating accuracy isotopic "clock" to zero.
The temperature at which this happens is known as the closure temperature or blocking temperature and is specific to a particular material and isotopic system. These temperatures are experimentally determined in the lab by artificially resetting sample minerals using a high-temperature furnace. As the mineral cools, the crystal structure begins to form and diffusion of isotopes is less easy.

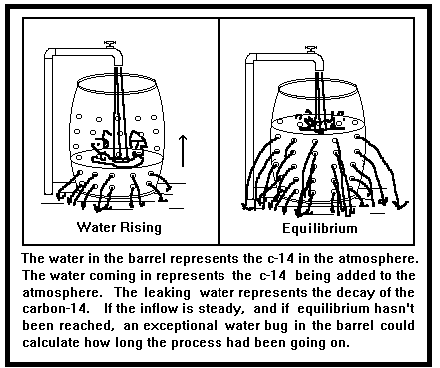
The basic equation of radiometric dating requires that neither the parent nuclide nor the daughter product. We would expect that radiometric dating, being allegedly so ' accurate,' would rescue the situation and provide exact ages for each of these hills. Apparently, this. The fossils occur in regular sequences time after time; radioactive decay happens, and repeated cross testing of radiometric dates confirms their validity. Radiometric Dating —Is it reliable? By: Arnold C. Mendez, Sr. There are many methods and techniques that geologists have used in the dating of the.








