Practical uses of carbon dating
09.05.2017
practical uses of carbon dating
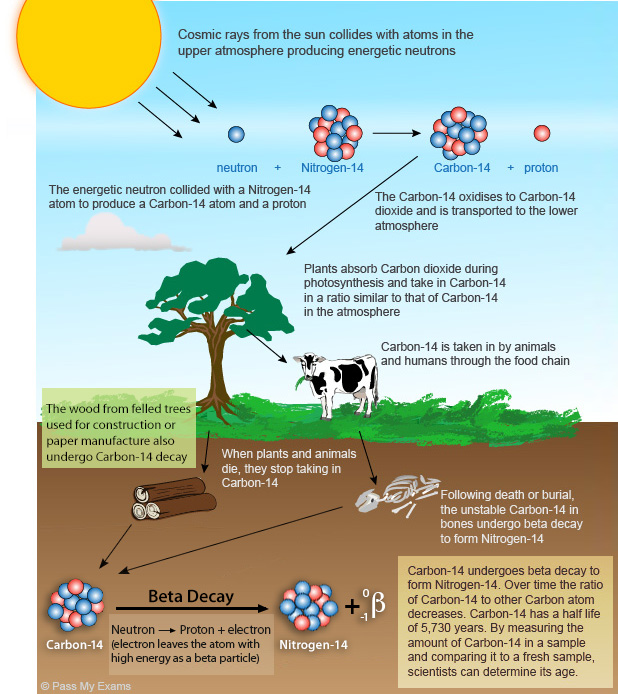
Carbon is constantly be generated in the atmosphere carrbon cycled through the carbon and rating cycles. Published on Sep 2, Carbon dating has shown that the cloth was made between and AD. What is the difference between iron ptactical steel? Korffthen employed at the Franklin Institute in Philadelphiathat the interaction of slow neutrons with 14 N in the upper atmosphere would create 14 C. For example, it is possible to determine the age of a person born after the s using the carbon content of teeth. INDEX Introduction Measurement Applications WWW Links k12 Publication Corrections Age calculation Calibration Pretreatment References Awards Credits What's new? Half of the remaining Carbon then decays over the next years carnon one fourth of the original amount. Conversely, nuclear testing increased the amount of 14 C in the atmosphere, which attained a maximum in of almost twice what it had been before the testing began. Describes radioactive half life and how to do some simple calculations using half life. This means that after 5, years, only half of the initial 14 C will remain; a quarter will remain after 11, years; an eighth after 17, years; and so on. The currently accepted value practical uses of carbon dating the dting of 14 C is 5, years. Practical uses of carbon dating Physics GCSE Biology GCSE Chemistry GCSE Mathematics. The technique of radiocarbon dating was developed by Willard Libby and his colleagues at the University of Chicago in

Recent EdSurge article on the Libretexts is just out - take a look. When we speak of the pracitcal Carbon, we adting often refer to the most naturally abundant stable isotope 12 C. Although 12 C is definitely essential to life, its unstable sister isotope 14 C has become of practical uses of carbon dating importance to the science world. Radiocarbon Dating is the process of determining the age of a sample by examining the amount of 14 C remaining against the known half-life, 5, years.
The practiczl this process works is because usds organisms are alive they are constantly replenishing their 14 C supply through respiration, providing them with a constant amount of the isotope. However, when an organism ceases to exist, it no longer takes in carbon from its environment and the unstable 14 C isotope begins to decay. From this science, we are able to approximate the date at which the organism were living on Earth.
Radiocarbon dating is used practical uses of carbon dating many fields to learn practical uses of carbon dating about the past conditions of organisms and the environments present on Earth. Radiocarbon dating usually referred to simply as carbon dating is a radiometric dating method. It uses the naturally occurring radioisotope pratical 14C to estimate the age of carbon-bearing materials up to about 58, to 62, years old.
Carbon has two stable, nonradioactive isotopes: There are also trace amounts of the unstable radioisotope carbon 14 C on Earth. Uees has a relatively short half-life of 5, years, datig that the fraction of carbon in a sample is halved over the course of 5, years due to radioactive decay to nitrogen The carbon isotope would vanish from Earth's atmosphere in less than a million years were it not for the constant influx of cosmic rays interacting with molecules of nitrogen N 2 and single nitrogen atoms N in the stratosphere.
Both processes of formation and decay of carbon are shown in Figure 1. Diagram of the formation of carbon forwardthe decay of carbon reverse. Carbon is constantly be generated in the atmosphere and cycled through the carbon and nitrogen cycles. Once an organism is decoupled from these cycles i. When plants fix atmospheric carbon dioxide CO 2 into organic compounds during photosynthesis, the resulting fraction of the isotope 14 C in the plant tissue will match the fraction of the isotope in the atmosphere and biosphere since they are coupled.
After a plants die, the incorporation of all carbon isotopes, including 14 C, stops and the concentration of 14 C declines due dating xiumin the radioactive decay of 14 C following. This follows first-order kinetics. The currently accepted value for the half-life of if C is 5, years.
This means that after 5, years, only half of the initial 14 C will remain; a quarter will remain after 11, years; an eighth after 17, years; and so on. The equation relating rate constant to half-life for first order kinetics is. In practical uses of carbon dating of the Dead Sea Scrolls were analyzed by carbon dating. From the measurement performed in the Dead Sea Scrolls were determined to be years old giving them a date of 53 BC, and confirming their authenticity.
Carbon dating datihg shown that the cloth was made between and AD. Thus, the Turin Shroud was made over a thousand years after the death of Jesus. Describes radioactive half life and how to do some simple calculations using half life. The technique of radiocarbon dating was developed by Willard Libby and his colleagues at the University of Chicago in Libby estimated that the steady-state radioactivity concentration of exchangeable carbon would be about 14 disintegrations per minute dpm per gram.
InLibby was awarded the Nobel Prize in chemistry for this work. He demonstrated the accuracy of radiocarbon dating by accurately estimating the age of wood from a series of samples for which the age was known, including an ancient Egyptian royal barge dating from BCE. Before Radiocarbon dating was able to be discovered, someone had to find the existence of the 14 C isotope. In Martin Kamen and Sam Ruben at the University of California, Berkeley Radiation Laboratory did just that.
They found a form, isotope, of Carbon that contained 8 neutrons and practical uses of carbon dating protons. Using this finding Willard Libby and his team at the University of Chicago proposed that Carbon was unstable and underwent a total of 14 disintegrations per minute per gram. Using this hypothesis, the initial half-life he determined was give or take 30 years.
Although it may be seen as outdated, many labs still use Libby's half-life in order to stay consistent in publications and calculations within the laboratory. From the discovery of Carbon to radiocarbon dating of fossils, we can see what an essential role Carbon has played and continues to play in our lives today. The entire process of Radiocarbon dating depends on the decay of carbon This process begins when an organism is no longer able to exchange Carbon with their environment.
Carbon is first formed when cosmic rays in the atmosphere allow for excess neutrons to be produced, which then react with Nitrogen to produce a constantly replenishing supply of carbon to exchange with organisms. Chemistry Biology Geology Mathematics Statistics Physics Social Sciences Engineering Medicine Agriculture Photosciences Humanities. Periodic Table of the Elements Reference Tables Physical Constants Units and Conversions Organic Chemistry Glossary. Search site Search Search.
Go back to previous article. Share Share Share Tweet Share. Skills to Develop Identify the age of materials that can be approximately determined using radiocarbon dating. The Carbon cycle Radiocarbon dating usually referred to simply as carbon dating is a radiometric dating method.

The development of this page will be gradual and contributions are invited. There are many, many interesting applications of radiocarbon dating in a variety of. Carbon is a radioactive isotope used to date organic material. Carbon dating works by comparing the amount of carbon in a sample to the amount of. Nuclear laboratories, awash with funds and prestige, spun off the discovery of an amazing new technique — radiocarbon dating. The radioactive isotope. Radiocarbon dating or in general radioisotopic dating method is used for estimating the age of old archaeological samples. For example, age of the earth, moon.








